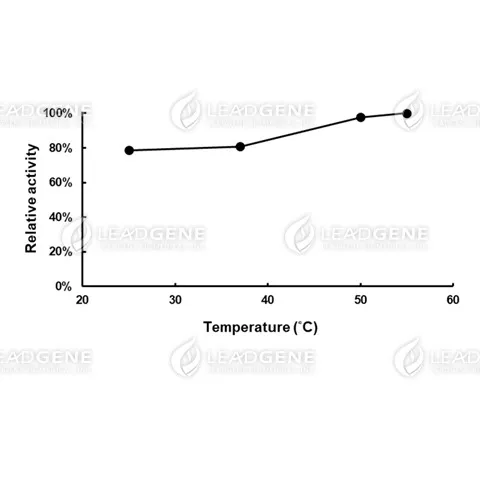
Description
Glucose dehydrogenase (GLD) (NAD(P)-dependent) is an enzyme that catalyzes the oxidation of glucose to gluconolactone while reducing the coenzymes NAD+ or NADP+ to NADH or NADPH. This enzyme is widely used in biosensors and diagnostic assays to measure blood glucose levels. Its high specificity and stability make it an essential tool in various biomedical applications.