|
The Discovery of Nanobodies |
Nanobodies (Nb), also known as single-domain antibodies (sdAb) or camelid antibodies, are unique antibody molecules derived from camelid species, such as camels and alpacas. These antibodies were first discovered in 1993 by Belgian scientists, who identified a type of antibody in camel serum consisting only of heavy chains, known as heavy-chain-only antibodies (HCAbs). HCAbs have a molecular weight of approximately 95 kDa. Structurally, their constant region contains crystallizable fragments (Fc) linked by disulfide bonds, including the hinge region and the dimeric CH2 and CH3 domains. In addition, HCAbs feature two variable regions (VHH, the VH domain of an HCAb). Nanobodies are derived from these variable regions, making them a distinct form of antibody fragment, as illustrated in Figure 1.
|
Structural Features of Nanobodies |
Nanobodies differ from single-chain variable fragments (scFv) in that they consist of only a single VHH domain and lack a light chain. This structural simplicity eliminates the need for linker peptides to connect heavy and light chains. With a molecular weight of merely 15 kDa—about one-tenth that of conventional antibodies—they offer distinct advantages, as illustrated in Figure 1.
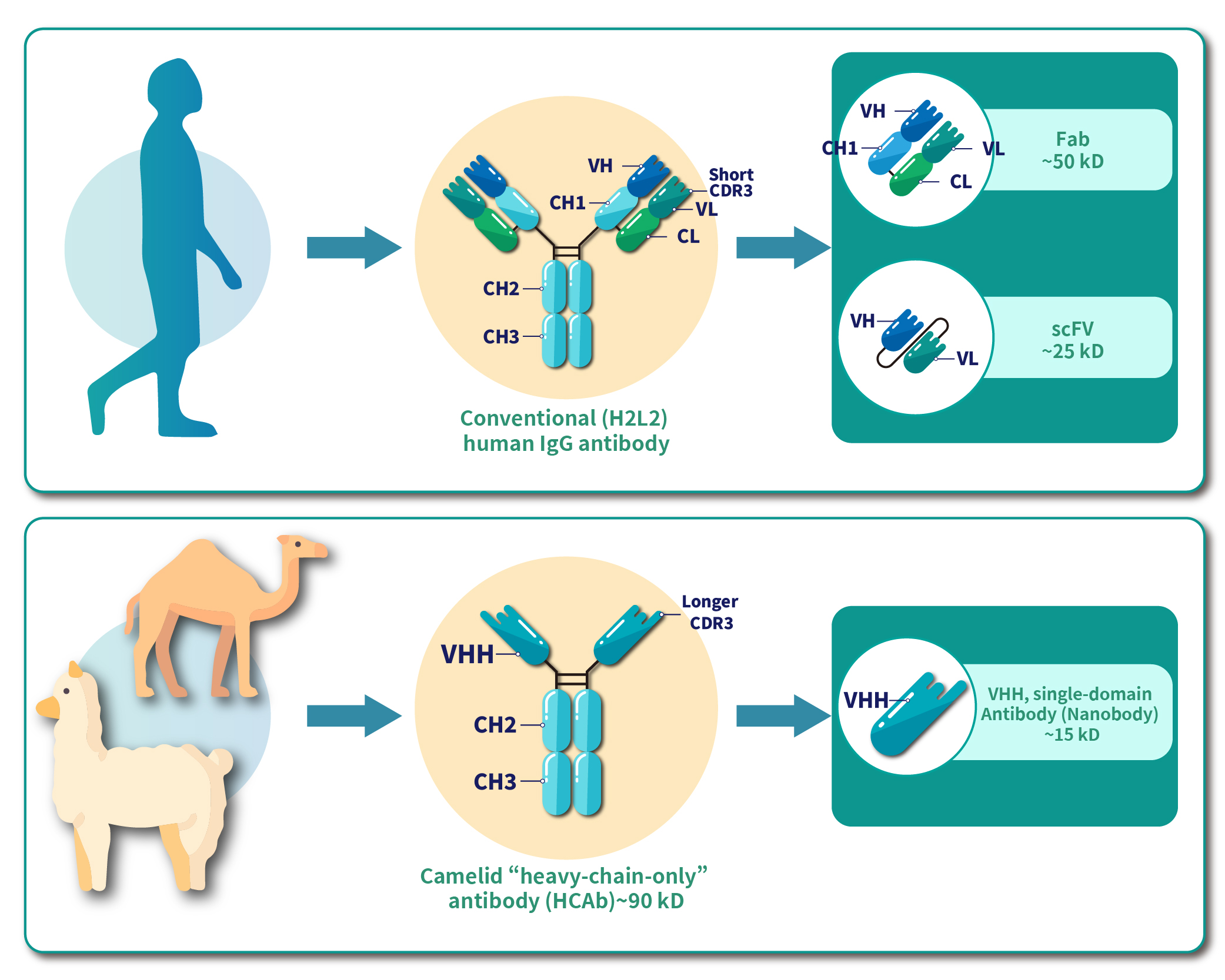
Figure 1. Structural differences between Conventional IgG antibody, Camelid antibody, and Nanobody.
In the shared structure between VHH nanobodies and the VH domains of conventional antibodies, there are notable differences beyond the conserved framework regions (FR1, FR2, FR3, and FR4) and complementarity-determining regions (CDR1, CDR2, and CDR3). The VH domain of conventional antibodies contains four highly conserved hydrophobic amino acids (V37, G44, L45, and W47) within FR2, whereas nanobodies replace these residues with hydrophilic amino acids (F37, E44, R45, and G47), significantly enhancing their solubility. Additionally, the CDR1 and CDR3 regions in nanobodies are generally longer than those in conventional VH domains, enabling them to target antigenic epitopes hidden within complex three-dimensional structures, such as enzyme active sites and viral receptor binding sites. Due to their unique structure and biochemical properties, nanobodies can precisely recognize and bind to these epitopes, greatly enhancing their potential in disease diagnosis and treatment, as illustrated in Figure 2.
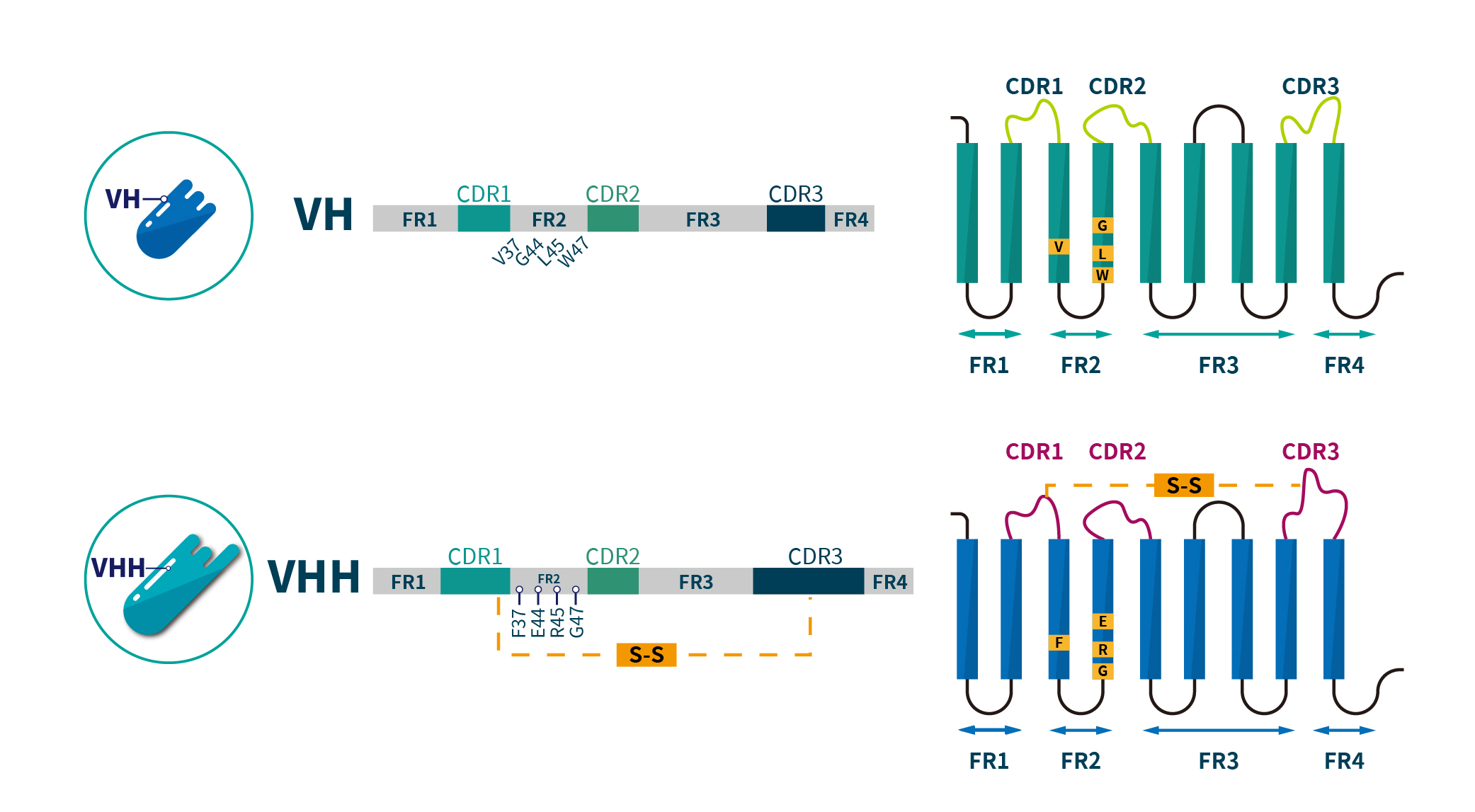
Figure 2. Structural differences between Conventional IgG antibody VH domain and Camelid VHH domain.
|
Advantages and Challenges of Nanobodies |
The unique structure of nanobodies (VHH antibodies) offers several advantages in the field of biomedicine, but they also present some challenges.
Advantages:
- Simple structure, easy expression, and development: Nanobodies consist of only a single variable heavy-chain domain (VHH) and lack light chains, which allows them to be efficiently produced in various expression systems, such as E. coli or yeast.
- Simple structure, easy expression, and development: Nanobodies consist of only a single variable heavy-chain domain (VHH) and lack light chains, which allows them to be efficiently produced in various expression systems, such as E. coli or yeast.
- High stability and strong affinity: Nanobodies are highly stable under extreme conditions, including variations in pH, temperature, and protease digestion. They also exhibit strong binding affinity toward their target antigens.
- High targeting capability and tissue penetration: Due to their small size, nanobodies can penetrate deep into tissues and even infiltrate the tumor microenvironment, making them highly advantageous for targeting tumor cells.
- Low immunogenicity and easy humanization: As nanobodies are derived from camelid species, they exhibit low immunogenicity in humans. Furthermore, they are relatively easy to humanize, enhancing their safety for clinical applications.
Challenges:
- Short half-life: Due to their small molecular weight, nanobodies typically have a shorter half-life, which may limit their effectiveness in certain clinical applications. However, this issue can be addressed by fusing nanobodies with serum albumin or Fc fragments to extend their half-life.
With their exceptional properties, as shown in Table 1. Nanobodies are becoming powerful tools in cancer diagnosis and therapy. However, future advancements must address technical challenges to fully unlock their potential across various applications.
Table 1. Comparison of the Characteristics of VHH Nanobodies and Conventional Antibodies.
|
|
Types of Nanobodies |
Based on their characteristics and applications, nanobodies can be categorized into the following types:Based on their characteristics and applications, nanobodies can be categorized into the following types:
- Natural Nanobody: These nanobodies are directly derived from the heavy-chain antibodies of camelid animals (e.g., camels, alpacas). They retain the natural structure and function of antibodies and are known for their high stability and low immunogenicity.
- Engineered Nanobody: Engineered nanobodies are modified versions of natural nanobodies, optimized through genetic engineering to enhance specific properties. For instance, their structure can be improved to achieve higher affinity, better stability, or greater specificity for particular antigens. These nanobodies are often used in more challenging biomedical applications, such as cancer treatment and precise diagnostics.
- Multivalent Nanobody: Multivalent nanobodies are complexes formed by combining multiple nanobodies. This configuration allows them to bind multiple antigens or multiple epitopes simultaneously, enhancing binding strength and therapeutic efficacy while reducing the chances of antigen escape. This type of nanobody is particularly useful in cancer immunotherapy.
- Bispecific Nanobody: Bispecific nanobodies consist of two nanobody fragments with different specificities, enabling them to recognize and bind two distinct antigens or epitopes. These nanobodies are valuable in cancer treatment and other complex diseases, as they can target multiple disease sites or regulate multiple signaling pathways simultaneously.
- Nanobody-Protein Fusion: Nanobody-protein fusions are formed by linking nanobodies with other functional molecules, such as enzymes, drugs, or therapeutic proteins. These fusion molecules combine targeting capabilities with therapeutic effects, making them suitable for precision therapy, targeted drug delivery, or enhanced immune response.
- Nanobody-Drug Conjugate (NDC): Nanobody-drug conjugates are complexes in which nanobodies are conjugated with cytotoxic drugs. These nanobodies precisely recognize and bind to cancer cells, delivering toxic drugs directly into the cells to achieve targeted tumor cell killing while minimizing damage to healthy cells.
These types of nanobodies have extensive potential in biomedical research, diagnostics, and therapy, as illustrated in Figure 3. With the continuous advancement of technology, more novel types of nanobodies are expected to be developed.
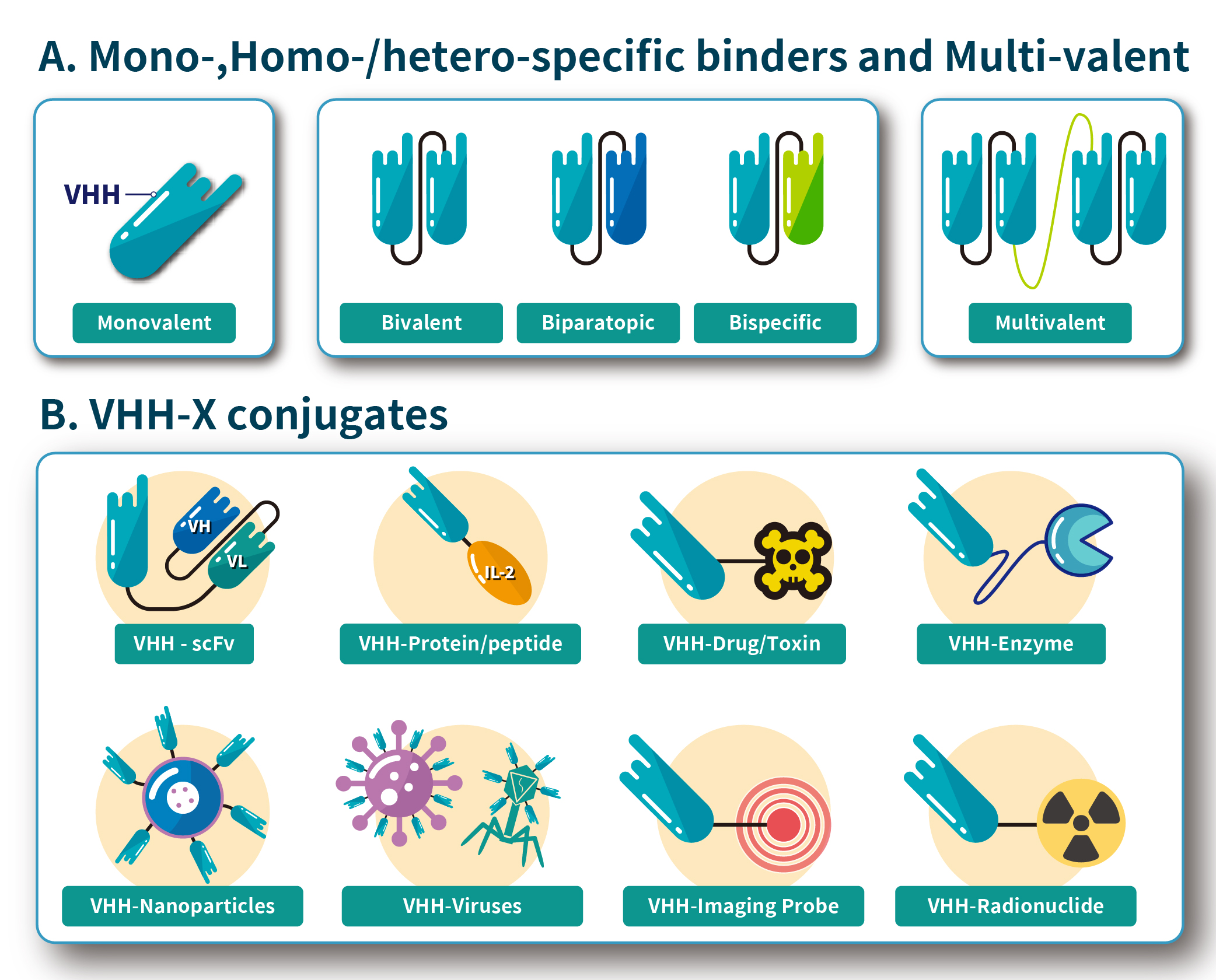
Figure 3. Overview of Nanobody Structure and Its Types.
|
Clinical Applications of Nanobodies |
Due to their exceptional properties, nanobodies have been widely applied in molecular imaging, diagnostics, drug delivery, tumor immunotherapy, and cellular therapies. Nanobodies have shown significant promise in treating central nervous system disorders, cardiovascular diseases, tumors, infectious diseases, and inflammatory conditions. In cancer therapy, several treatment modalities, including bispecific nanobodies, nanobody-drug conjugates (NDC), CAR-T, CAR-NK, and radiopharmaceuticals, have been developed, offering new hope for patients.
As of July 2023, at least four nanobody-based drugs have been approved globally. Caplacizumab (Cablivi®), developed by Ablynx, was the first nanobody drug approved for the treatment of acquired thrombotic thrombocytopenic purpura (aTTP). It received European Medicines Agency approval on August 31, 2018. Ciltacabtagene Autoleucel (CARVYKTI), a CAR-T cell product developed by Legend Biotech, utilizes a unique bivalent nanobody design and was the first FDA-approved CAR-T therapy based on VHH nanobodies for the treatment of relapsed or refractory multiple myeloma. Another nanobody drug, Ozoralizumab, developed by Ablynx, is a humanized trivalent bispecific nanobody containing two anti-TNF-α nanobodies and one anti-human serum albumin nanobody. It was approved in Japan on September 26, 2022. Envafolimab, developed by Tracon Pharmaceuticals and 3D Medicines, is a PD-L1 single-domain antibody fused with an Fc region, approved in China in November 2021, as shown in Figure 4 and Table 2.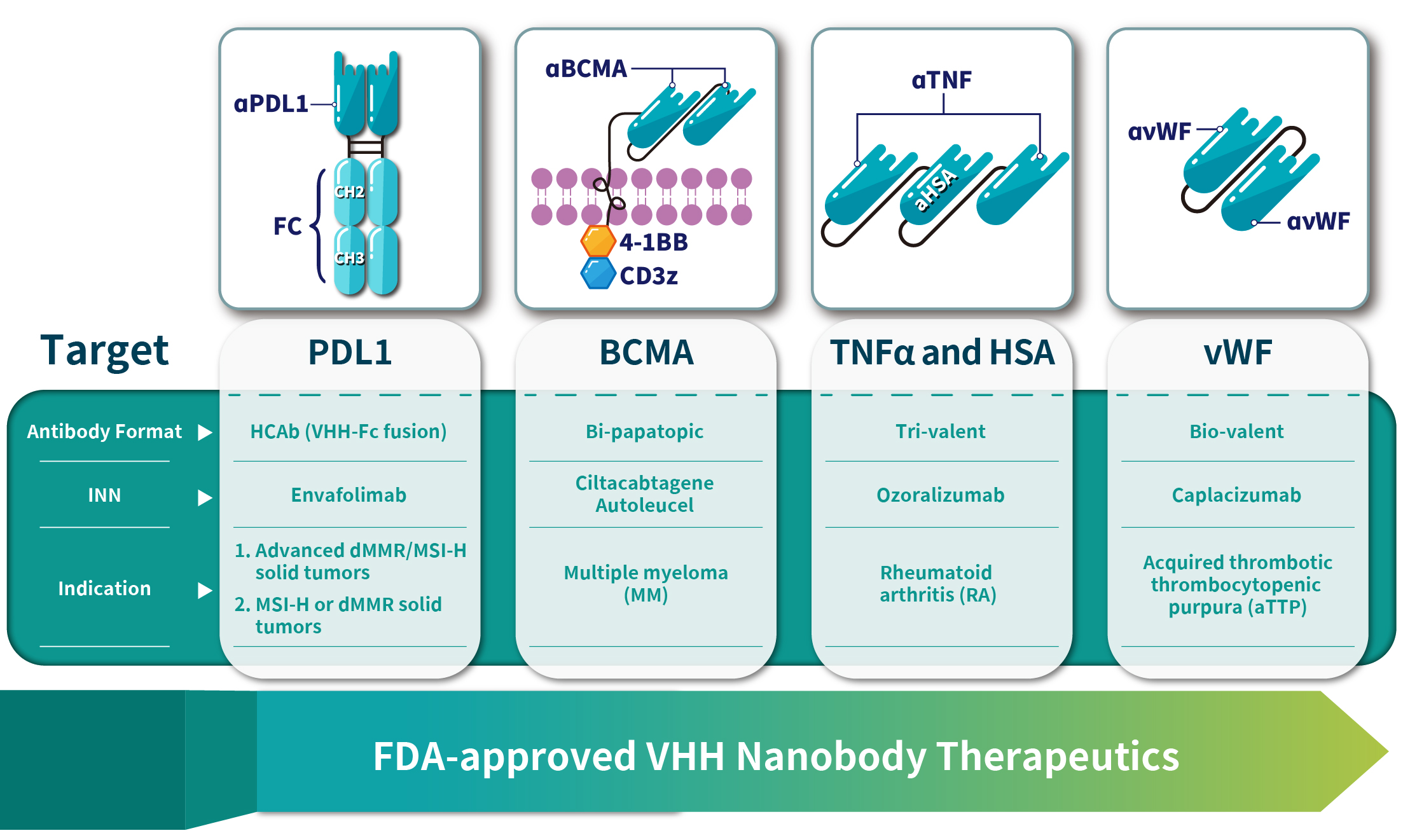 Figure 4: Four Successfully Marketed Nanobody Drugs.
Figure 4: Four Successfully Marketed Nanobody Drugs.
Table 2. Approved Nanobody Drugs.
|
In addition, more than 20 nanobody-related drugs have entered clinical stages. Based on the latest clinical research developments, Table 3 provides detailed information on various nanobody drugs, including their clinical trial phases and indications. These nanobody drugs cover a wide range of applications, from solid tumors to autoimmune inflammatory diseases, and from hematological disorders to immune dysregulation diseases, further demonstrating the vast potential of nanobodies in the medical field. As clinical research progresses, more nanobody drugs are expected to drive the development of related therapeutic approaches, offering patients greater treatment opportunities and hope.
Table 3. Nanobody Drugs in Clinical Trials.
|
|
Nanobody Applications in Medical Diagnosis and Research |
As technology continues to advance, the application scope of nanobodies will further expand. In the field of therapeutic antibodies, VHH nanobodies are expected to become the next generation of targeted therapeutic tools, particularly for treating cancers and inflammatory diseases. Their high specificity, low immunogenicity, and deep tissue penetration capability make them a promising direction for future therapeutic and diagnostic advancements.
|
Global Market Size of VHH Nanobodies |
VHH nanobodies, with their unique biological properties and broad application prospects, have become an essential innovation in the field of biopharmaceuticals.
According to market research reports, the global VHH nanobody market was valued at approximately $600 million in 2021 and is expected to grow to $2 billion by 2026, with a compound annual growth rate (CAGR) of about 25%. This rapid growth reflects the increasing demand for VHH nanobodies in drug development, diagnostic reagents, and research tools. Moreover, the investments and acquisitions by biotechnology companies in the VHH nanobody field highlight their high economic potential. For example, Sanofi’s acquisition of Ablynx, a leader in the VHH nanobody space, for €3.9 billion underscores the commercial value of VHH technology.
One of the key indicators of the economic value of VHH nanobodies is the success of Caplacizumab (Cablivi), the first VHH-based drug approved by the FDA, which has shown excellent market performance globally. Its sales revenue serves as a critical benchmark for measuring the economic potential of VHH nanobody technology.
As more VHH-based drugs enter clinical trials and receive regulatory approvals, the VHH nanobody market is expected to continue expanding. Overall, VHH nanobodies occupy a prominent position in the global biopharmaceutical market due to their unique advantages. The market is projected to maintain rapid growth in the coming years, delivering significant economic value to the global economy.
|
Leadgene Service |
|
References |
- Hamers-Casterman, C., Desmyter, A., Maes, E., Verbrugghe, A., Hamers, R., Songa, E. B., ... & Muyldermans, S. (1993). Naturally occurring antibodies devoid of light chains. Nature, 363(6428), 446–448.
- Harmsen, M. M., & De Haard, H. J. (2007). Properties, production, and applications of camelid single-domain antibody fragments. Applied Microbiology and Biotechnology, 77(1), 13–22.
- Lauwereys, M., Arbabi Ghahroudi, M., Desmyter, A., Kinne, J., Holzer, W., De Genst, E., ... & Muyldermans, S. (1998). Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO Journal, 17(13), 3512–3520.
- Muyldermans, S. (2013). Nanobodies: Natural single-domain antibodies. Annual Review of Biochemistry, 82, 775–797.
- Vincke, C., Loris, R., Saerens, D., Martinez-Rodriguez, S., Muyldermans, S., & Conrath, K. (2012). General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. Journal of Biological Chemistry, 287(10), 7346–7355.
- Wesolowski, J., Alzogaray, V., Reyelt, J., Unger, M., Juarez, K., Urrutia, M., ... & Steindl, F. (2009). Single-domain antibodies: Promising experimental and therapeutic tools in infection and immunity. Medical Microbiology and Immunology, 198, 157–174.
- Wesolowski, J., Jain, N., & Scheid, P. (2009). Single-domain antibodies: Promises and challenges of their clinical development. Expert Opinion on Biological Therapy, 9(8), 109–117.
