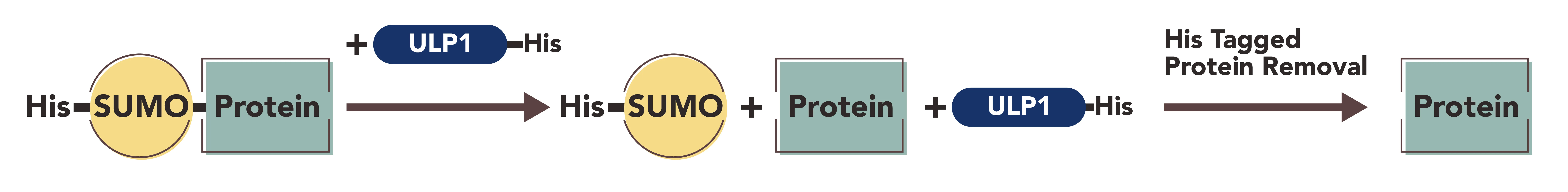
The SUMO tag has become a popular biotechnological tool due to its numerous advantages in protein expression and production, such as improved solubility, stability, folding, and crystallography. However, in downstream processes, it's crucial to remove the SUMO tag from the protein of interest.
This can be achieved by using ULP1, a SUMO-specific protease, followed by a purification process.
To make the purification process of the target protein expressed in E. coli more convenient, Leadgene has a 6xHis-fused ULP1 protease that identifies and cleaves the SUMO tag C-terminally of a conserved Gly-Gly motif, releasing the target protein from the SUMO tag.
The target protein can then be quickly purified with a single step of His-tag purification.