Numerous advantages of freeze-drying reagents have been documented, with the most significant one of all being the ability to sustain the ingredients‘ activities at room temperature.
This process requires highly professional and repetitionary-condition optimizations.
At Leadgene, our innovative LEADSPHERE® lyophilization technology serves as a platform to ease the development of your reagents in lyophilized and in stable sphere format while maintaining the components’ bioactive condition at room temperature for at least 12 months.
 |
|
||
 |
|
||
|
Molecules that can be used in LEADSPHERE® technology: |
Antibodies, Proteins, Enzymes, Chemicals, PCR Mixture
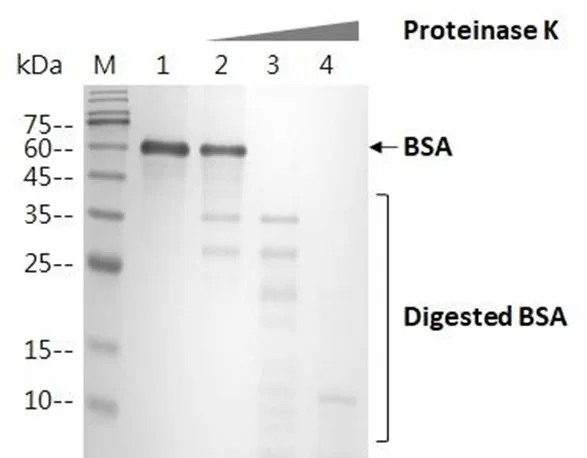
Employing an endopeptidase, LEADSPHERE® Proteinase K, used for DNA or RNA sample preparation by digesting proteins and inactivating ribonuclease is a notable example of LEADSPHERE® lyophilization technology.
LEADSPHERE® Proteinase K, provided in a lyophilized sphere format, not only has all the advantages of LEADSPHERE® technology, such as product stability and high-temperature tolerance, but it also has other benefits in multiple aspects:
1. Supports a “pick and place” automation system for commercial-scale production.
2. Reduces pipetting step and simplifies protocols for end users.
3. Allows direct mixing with various sample types such as saliva, blood, or samples in trace quantities.
 |
|
||
LEADSPHERE® Proteinase K formulation is fine-tuned for direct mixing with and digestion of saliva. LEADSPHERE® Proteinase K is generated with an ISO 13485 quality management system specifically for medical devices and has been thoroughly used in applications to identify viruses, including SARS-CoV-2.
