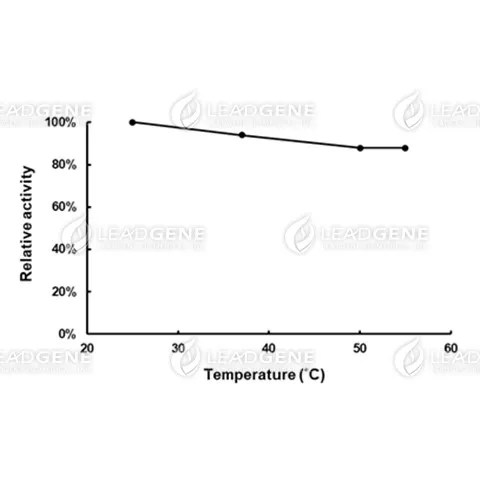
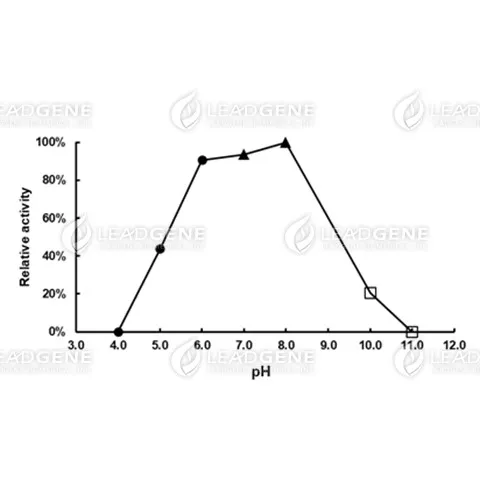
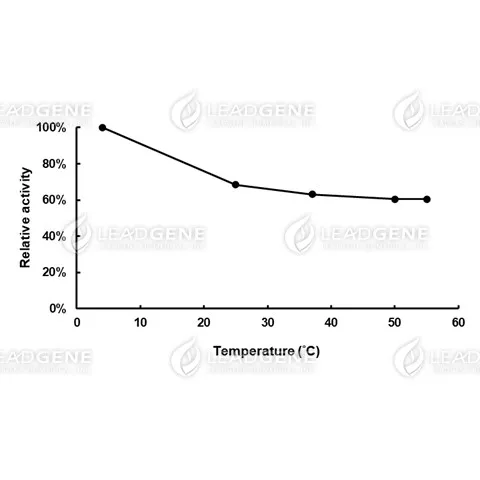
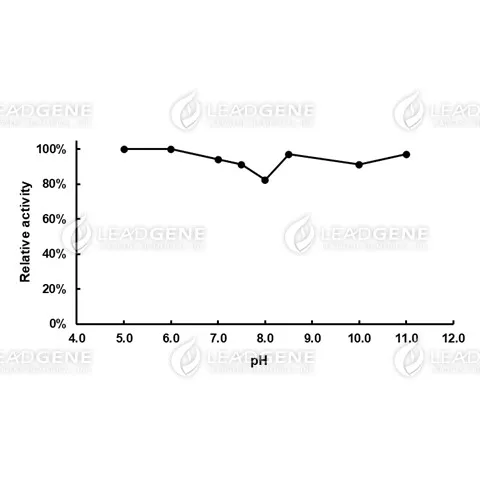
Description
Cholesterol oxidase (CO) is a bacterial enzyme that catalyzes the oxidation of cholesterol to cholest-4-en-3-one, producing hydrogen peroxide as a byproduct. This enzyme is widely used in clinical diagnostics for measuring cholesterol levels in blood samples, as it plays a crucial role in cholesterol metabolism. Additionally, cholesterol oxidase is used in research to study cholesterol biosynthesis, and in the biotechnology industry for the production of steroids and as a biocatalyst in biosensors. It is primarily sourced from species such as Streptomyces and Brevibacterium.