-
Species of Origin
Human
Expression system
Escherichia coli
-
Affinity Tag
His Tag (C-term)
Buffer
Lyophilized from a 0.2 µm filtered solution of PBS, pH 8.0.
-
Purity
>97% as determined by SDS-PAGE analysis.
Molecular weight
The protein has a calculated MW of 17.7 kDa.
The protein migrates as 17 kDa under reducing condition (SDS-PAGE analysis). -
Activity
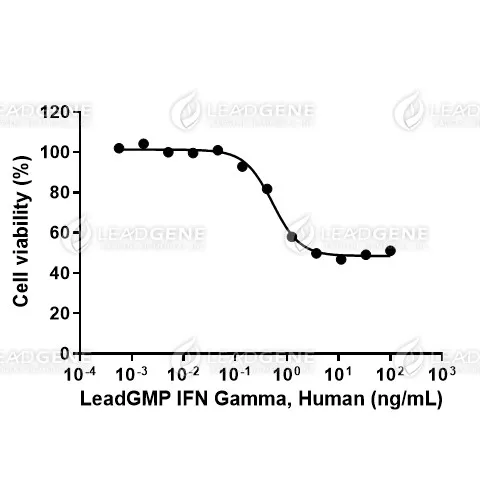
Measure by its ability to induce cytotoxicity in HT29 cells. The ED₅₀ for this effect is <1 ng/mL. The specific activity of recombinant human IFN gamma is approximately >1 x 10⁷ IU/mg, which is calibrated against the human IFN Gamma WHO Reference Material (NIBSC code: 87/586).
Endotoxin level
<0.05 EU per 1 µg of the protein by the LAL method.
-
Mycoplasma
Not detected
Form
Lyophilized
-
Specifications
-
Background
-
Background
The cytokine IFN gamma could protect cells from viral infections and belongs to the family of interferons. A lot of studies have shown that IFN gamma secreted by antigen triggered cell types, including T cells, naive CD4+ T cells, macrophages, dendritic cells, and B cells. IFN gamma plays an important role to trigger the macrophage act against a diverse group of microbial targets, and the pleiotropic molecule associated with antiproliferative, pro-apoptotic and antitumor mechanisms. Based on the effector cytokine considered as a major effector of immunity, it has been used in the treatment of several diseases.
Synonyms
Interferon gamma, Immune interferon
-
Uniprot ID
P01579
Sequence Note
Gln24-Gln166
-
-
Instruction
-
Reconstitution
It is recommended to reconstitute the lyophilized protein in sterile H₂O to a concentration not less than 0.5 mg/mL and incubate the stock solution for at least 20 min to ensure sufficient re-dissolved.
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at -20°C or lower for long term storage.
-
Stability & Storage
This product is stable after storage at:
- -20°C for 12 months in lyophilized state from date of receipt.
- -20°C or -80°C for 1 month under sterile conditions after reconstitution.
Avoid repeated freeze/thaw cycles.
Manufacturing specifications
LeadGMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP certified facility. The processes include:
- Animal-free reagent and laboratory
- Manufactured and tested under GMP guideline
- Testing and traceability of raw material
- Records of the maintenance and equipment calibration
- Personnel training records
- Batch-to-batch consistency
- Documentation of QA control and process changes
- Manufactured and tested under an ISO 13485:2016 certified quality management system
- Stability monitor of product shelf-life
-
-
Image
1/2 -
Review

Help others learn more about this product. Use the link below to share your experience.
-
Publication

There are currently no publications. Use the link below to let us know.
-
Datasheet & Documents
1/1
Disclaimer:For Research Use or Further Manufacturing Only.