-
Species of Origin
Human
Expression system
Escherichia coli
-
Affinity Tag
His Tag (C-term)
Buffer
Lyophilized from a 0.2 µm filtered solution of NaPi buffer, 0.018% SDS, pH 7.5.
-
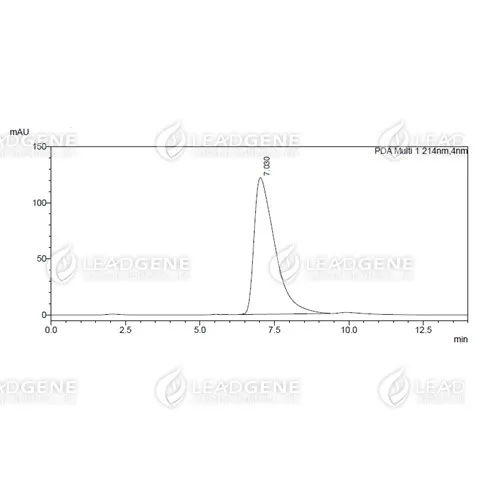
Purity
>97% as determined by SDS-PAGE analysis.
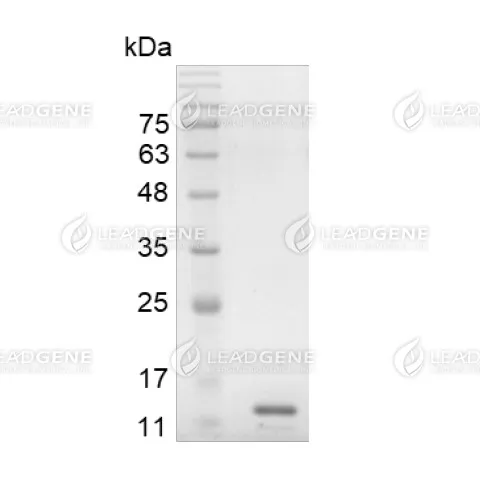
Molecular weight
The protein has a calculated MW of 16.4 kDa.
The protein migrates as 13 kDa under reducing condition (SDS-PAGE analysis). -
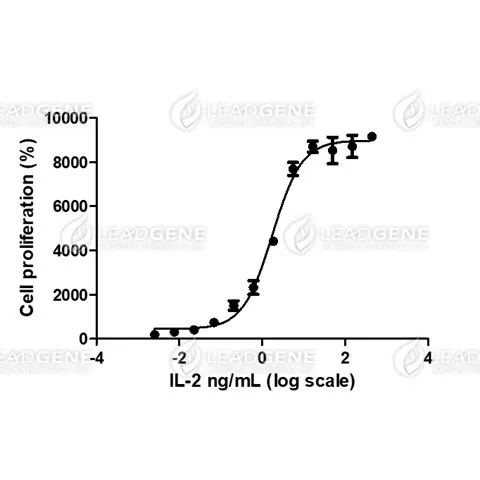
Activity
Measure by its ability to induce proliferation in CTLL-2 cells. The ED₅₀ for this effect is <3 ng/mL. The specific activity of recombinant human IL-2 is >5 x 10⁶ IU/mg, which is calibrated against the human IL-2 WHO International Standard (NIBSC code: 86/500).
Endotoxin level
<0.05 EU per 1 µg of the protein by the LAL method.
-
Mycoplasma
Not detected
Form
Lyophilized
-
Specifications
-
Background
-
Background
Interleukin-2 (IL-2) is a 15.5 kDa four bundle, immuno-modulatory cytokine which is expressed predominately by antigen-simulated CD4+ T cells, CD8+ T cells, natural killer (NK) cells, and dendritic cells (DC). IL-2 plays a fundamental role in promoting NK cell proliferation, cytotoxicity, and B cells differentiation. IL-2 modulate T cell differentiation programs, promoting naïve CD4+ T cell differentiation into T helper-1 (Th1) and T helper-2 (Th2) cells.
Synonyms
Interleukin-2, T-cell growth factor , TCGF
-
Uniprot ID
P60568
Sequence Note
Ala21-Thr153
-
-
Instruction
-
Reconstitution
It is recommended to reconstitute the lyophilized protein in sterile H₂O to a concentration not less than 0.5 mg/mL and incubate the stock solution for at least 20 min to ensure sufficient re-dissolved.
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at -20°C or lower for long term storage.
-
Stability & Storage
This product is stable after storage at:
- -20°C for 12 months in lyophilized state from date of receipt.
- -20°C or -80°C for 1 month under sterile conditions after reconstitution.
Avoid repeated freeze/thaw cycles.
Manufacturing specifications
LeadGMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP certified facility. The processes include:
- Animal-free reagent and laboratory
- Manufactured and tested under GMP guideline
- Testing and traceability of raw material
- Records of the maintenance and equipment calibration
- Personnel training records
- Batch-to-batch consistency
- Documentation of QA control and process changes
- Manufactured and tested under an ISO 13485:2016 certified quality management system
- Stability monitor of product shelf-life
-
-
Image
1/3 -
Review

Help others learn more about this product. Use the link below to share your experience.
-
Publication

There are currently no publications. Use the link below to let us know.
-
Datasheet & Documents
1/1
Disclaimer:For Research Use or Further Manufacturing Only.