-
Species of Origin
Human
Expression system
Escherichia coli
-
Affinity Tag
His Tag (C-term)
Buffer
Lyophilized from a 0.2 µm filtered solution of 500 mM NaCl PBS, pH 7.4.
-
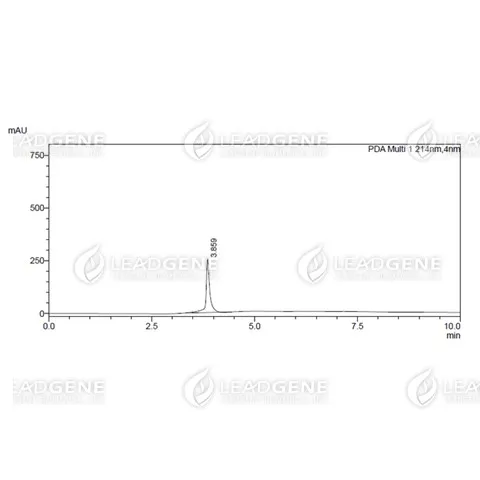
Purity
>97% as determined by SDS-PAGE analysis.
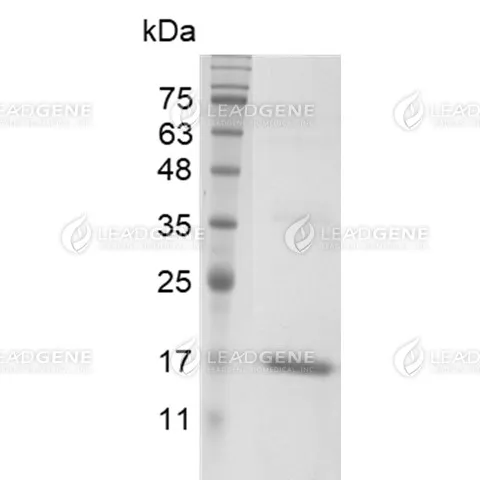
Molecular weight
The protein has a calculated MW of 18.3 kDa.
The protein migrates as 17 kDa under reducing condition (SDS-PAGE analysis). -
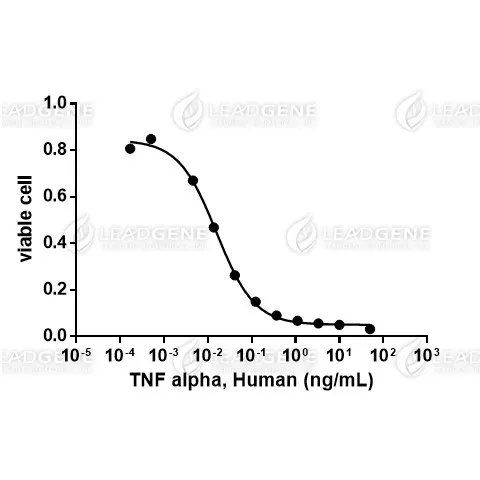
Activity
Measure by its ability to induce cytotoxicity in L929 cells in the presence of actinomycin D. The ED₅₀ for this effect is < 0.1 ng/mL. The specific activity of recombinant human TNF alpha is approximately ≧ 1 x 10⁷ IU/mg, which is calibrated against the human TNF Alpha WHO International Standard (NIBSC code: 12/154).
Endotoxin level
<0.05 EU per 1 µg of the protein by the LAL method.
-
Mycoplasma
Not detected
Form
Lyophilized
-
Specifications
-
Background
-
Background
Tumor necrosis factor alpha (TNF alpha) stimulates the acute phase of the immune response. In response to a pathogen, TNF alpha is one of the first to be released and can apply its effects in many organs. TNF alpha stimulates the release of corticotropic releasing hormone, suppresses appetite, and induces fever, in the hypothalamus. TNF increase vasodilation and loss of vascular permeability, it helps recruit lymphocyte, neutrophil, and monocyte to the inflammation site by regulating chemokine release.
Synonyms
Tumor necrosis factor, Cachectin, Tumor necrosis factor ligand superfamily member 2 , TNF-a
-
Uniprot ID
P01375
Sequence Note
Val77-Leu233
-
-
Instruction
-
Reconstitution
It is recommended to reconstitute the lyophilized protein in sterile H₂O to a concentration not less than 0.5 mg/mL and incubate the stock solution for at least 20 min to ensure sufficient re-dissolved.
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at -20°C or lower for long term storage.
-
Stability & Storage
This product is stable after storage at:
- -20°C for 12 months in lyophilized state from date of receipt.
- -20°C or -80°C for 1 month under sterile conditions after reconstitution.
Avoid repeated freeze/thaw cycles.
Manufacturing specifications
LeadGMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP certified facility. The processes include:
- Animal-free reagent and laboratory
- Manufactured and tested under GMP guideline
- Testing and traceability of raw material
- Records of the maintenance and equipment calibration
- Personnel training records
- Batch-to-batch consistency
- Documentation of QA control and process changes
- Manufactured and tested under an ISO 13485:2016 certified quality management system
- Stability monitor of product shelf-life
-
-
Image
1/3 -
Review

Help others learn more about this product. Use the link below to share your experience.
-
Publication

There are currently no publications. Use the link below to let us know.
-
Datasheet & Documents
1/1
Disclaimer:For Research Use or Further Manufacturing Only.