-
Species of Origin
Yeast
Expression system
Escherichia coli
-
Concentration
0.1 U/ μL
Buffer
20 mM Tris-HCl, 100 mM NaCl, 1 mM DTT, 0.1 mM EDTA, 50% Glycerol, pH 8.0
-
Purity
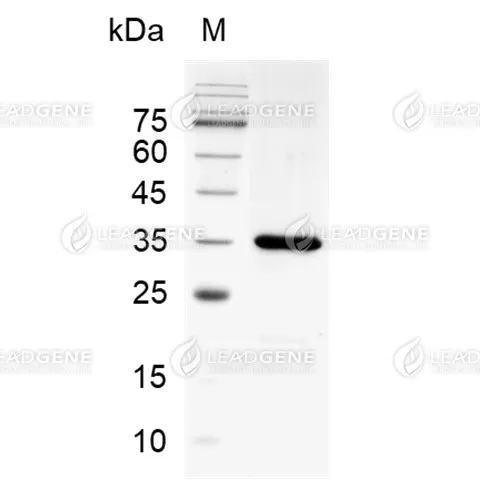
>95% as determined by SDS-PAGE analysis.
Molecular weight
The protein has a calculated MW of 33 kDa.
The protein migrates as 35 kDa under reducing condition (SDS-PAGE analysis). -
Activity
One unit is the amount of enzyme that will generate 1 µmol of phosphate per minute from inorganic pyrophosphate (ppi) under standard reaction conditions
(a 10 minute reaction at 25°C in 100 mM Tris-HCl, 2 mM MgCl2 and 2 mM PPi in a reaction volume of 0.5 mL, pH7.2).Endotoxin level
<0.1 EU per 1 μg of the protein by the LAL method.
-
Mycoplasma
Not detected
Form
Liquid
-
Specifications
-
Background
-
Background
Inorganic pyrophosphatase (PPase) is an enzyme that catalyzes the hydrolysis of inorganic pyrophosphate (PPi) into two phosphate ions. This reaction is crucial for cellular phosphate metabolism and energy balance. In humans, the PPA1 gene encodes this enzyme, which is ubiquitously expressed across various tissues. PPases are essential in processes such as DNA synthesis, lipid metabolism, and calcium absorption. They are found in both prokaryotic and eukaryotic organisms, highlighting their fundamental role in biology.
-
-
Instruction
-
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at -20°C or lower for long term storage.
Stability & Storage
This product is stable after storage at:
● -20°C for long-term storage under sterile conditions. Avoid repeated free-thaw cycles. -
Manufacturing specifications
LeadGMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP certified facility. The processes include:
- Animal-free reagent and laboratory
- Manufactured and tested under GMP guideline
- Testing and traceability of raw material
- Records of the maintenance and equipment calibration
- Personnel training records
- Batch-to-batch consistency
- Documentation of QA control and process changes
- Manufactured and tested under an ISO 13485:2016 certified quality management system
- Stability monitor of product shelf-life
-
-
Image
1/1 -
Review

Help others learn more about this product. Use the link below to share your experience.
-
Publication

There are currently no publications. Use the link below to let us know.
-
Datasheet & Documents
1/1