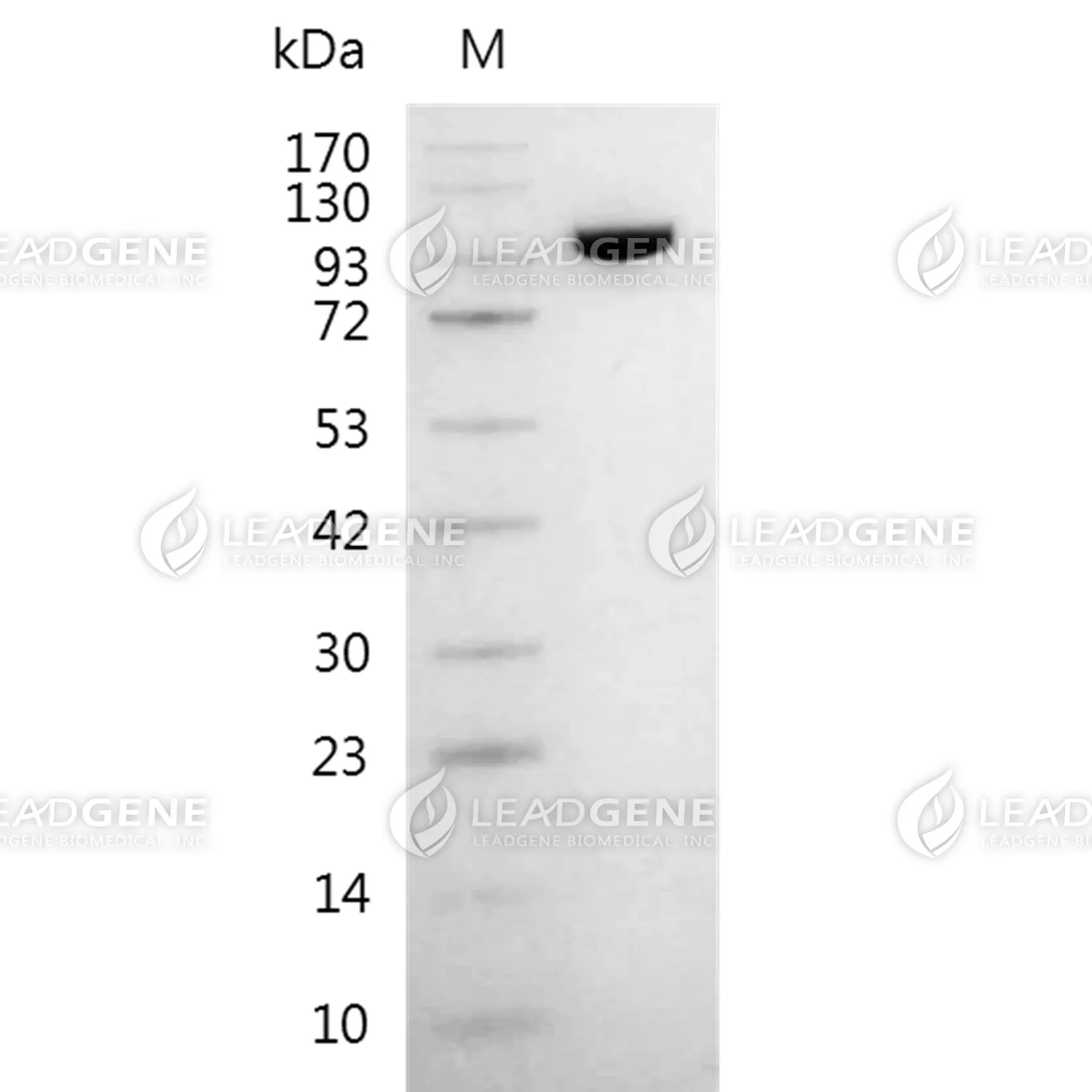
Description
Bacteriophage T7 RNA Polymerase is a DNA-dependent RNA polymerase with high specificity for the T7 promoter. This enzyme catalyzes the 5’→3’ synthesis of RNA from DNA downstream from its promoter.
Components
25,000 U
Items
T7 RNA Polymerase (200 U/μL)
Quantity
1 vial (25,000 U)
Items
10× RNA Polymerase reaction buffer
Quantity
1 vial (1 mL)
Items
100 mM DTT
Quantity
1 vial (1 mL)